Pharmaceutics | Free Full-Text | Application and Multi-Stage Optimization of Daylight Polymer 3D Printing of Personalized Medicine Products
Updates of Ph. Eur. dosage form monographs and general chapters – Users invited to comment in Pharmeuropa 33.1 - European Directorate for the Quality of Medicines & HealthCare

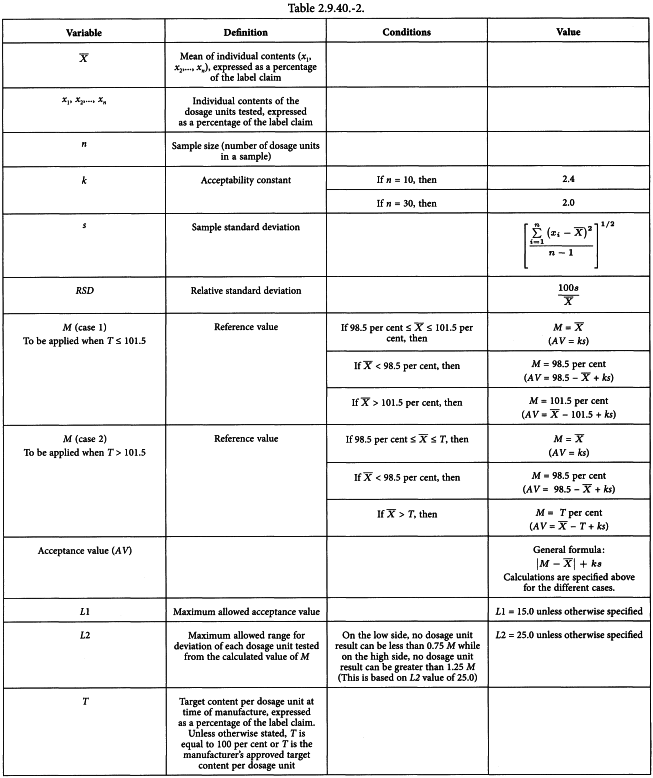
Pharmacopoeial quality control - Eur. 2.9) Uniformity of content (BP Appendix XII H, Ph. Eur. 2.9) - Studocu
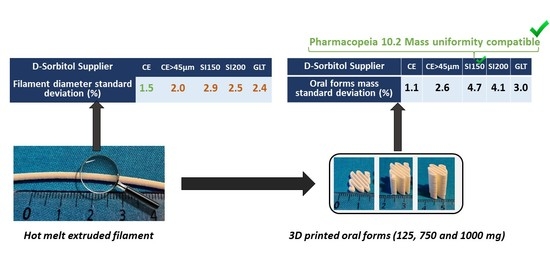
Molecules | Free Full-Text | D-Sorbitol Physical Properties Effects on Filaments Used by 3D Printing Process for Personalized Medicine
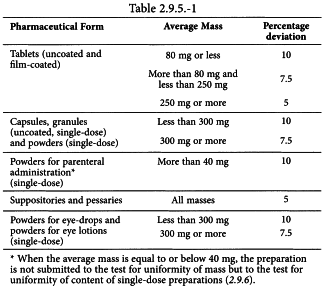
2.9.5. UNIFORMITY OF MASS OF SINGLE-DOSE PREPARATIONS 2.9.6. UNIFORMITY OF CONTENT OF SINGLE-DOSE PREPARATIONS

2.9.6. UNIFORMITY OF CONTENT OF SINGLE-DOSE … / 2-9-6-uniformity-of-content-of-single-dose.pdf / PDF4PRO